Ten patients having split-thickness skin grafting for burn injury were treated with the fish skin xenografts.
Abstract
Methods
Results
There were no adverse reactions noted on the use of the fish skin grafts. No patient had any reaction to the fish skin and there was a zero incidence of infection. The handling of the fish skin was excellent, a robust and pliable xenograft that was easy to apply.
The quality of donor site healing was judged to be good in all cases. Both the analgesic effect noted and the relatively short average times until 100% re-epithelialization are promising. We also illustrate two cases where the dressing was used to treat superficial burns.
INTRODUCTION
Early excision and application of Split Skin Grafting is the main stay of treatment of deep dermal and full thickness burn injury to avoid common complications like sepsis, multi-organ failure, and acute kidney injury.1 When treating large burns, autologous skin availability becomes a problem and burn surgeons often rely heavily on allogenic and xenogeneic skin for temporary coverage after excision. Human cadaver and pig skin are major sources of this temporary coverage. Application of cadaveric and pig skin grafts carries a risk of auto-immune response and risk of viral and bacterial diseases transmission,2 and there are many cultural and religious rejections for use of porcine grafts in many Muslim countries.3 Cadaveric skin is in limited supply and is understandably very expensive.
There has recently become available an alternative resource of xenograft using acellular fish skin (Kerecis Omega3 Burn). This has been described as providing an effective, safe, efficient skin substitute, free of the risk of transmission of viral disease,4 and auto-immune reaction risk.3,5 Furthermore, acellular fish skin has also been used with significant success in the healing process of acute6 and hard to heal wounds like diabetic foot ulcer, and chronic non-healing leg wounds of other varieties.3
Despite being separated by over 350 million years of evolution, fish skin has great similarity to mammalian skin due to its conserved protein structure.7 The skin of cold-sea adapted species like the Atlantic cod is, however, far richer in omega-3 polyunsaturated fatty acids (PUFAs) than warm-sea adapted species. It has a porous microstructure8,9 and has been described as being useful to cover exposed tendon and bone,10,11 initiating granulation of the exposed wound bed to kickstart a stagnated healing process.
The minimal processing required in the manufacturing of fish skin maintains its three-dimensional structure, as well as its anti-inflammatory and anti-infective properties. Fish species indeed live in an aquatic environment substantially richer in pathogens compared to the aerial environment of humans.12 Fish skin is rich in Omega3 PUFAs, eicosapentaenoic acid, and docosahexaenoic acid13 which are highly effective as antimicrobial agents and in modulating the inflammatory response of the acute wound healing stage. The fish skin is stored at room temperature, has a shelf life of 3 years and is marketed as an off-the-shelf product.6 Due to these properties, fish skin is an ideal choice for the treatment of combat casualties at Field Hospital level, where cadaver skin or pig skin are not practical to use due to their short shelf life and cold chain issues.4
Due to these properties, we started using the acellular fish skin on our burn casualties at Burn Centre of the Queen Elizabeth Hospital Birmingham, one of the Major Burns Centres in the UK, as a pilot to inform future randomized controlled studies.
SPLIT THICKNESS SKIN GRAFT HARVESTING PROCEDURE
Ten patients having split-thickness skin grafting for burn injury were treated with the fish skin xenografts. All patients were over 18 years of age. All donor sites were harvested at a depth of 8/1,000th of an inch. After soaking of the fish skin in saline, the fish skin was applied and held in place with a secondary dressing of dry gauze. All skin graft donor sites for each patient were thus treated. The first dressing change to the donor site after surgery was performed at 7 days and then was performed every 3 days thereafter until fully healed. The symptoms and signs of infection were assessed, and pain was assessed using a Verbal rating Score of 0–10 at each dressing change. Days to 90% epithelialization and to 100% epithelialization were recorded, as assessed visually by the senior author.
RESULTS
Ten patients were included in the donor site study. The average age of the patients was 45 (range 19–80), which included six males and four females. The size of the donor sites ranged from 40 cm2 to 950 cm2. Time to 90% epithelialization was reached with an average of 8.5 days (range 7–13). Time to 100% epithelialization had an average of 11.5 days (range 10–16) (Fig. 1). The patient that had the longest time until 100% re-epithelialization was the patient with the largest surface area of donor site (950 cm2) and had the most severe burn injuries.
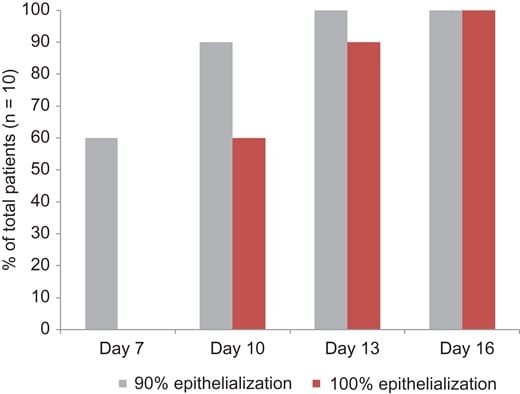
FIGURE 1. Time in days until re-epithelialization of donor site wounds. Gray bars represent 90% and red bars complete re-epithelialization.
There were no adverse reactions noted on the use of the fish skin grafts. No patient had any reaction to the fish skin and there was a zero incidence of infection of the wounds. The handling of the fish skin was excellent, a robust and pliable xenograft that was easy to apply.
The quality of donor site healing was judged to be good in all cases (Fig. 2).
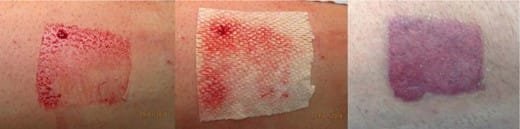
FIGURE 2.Left: Donor site to front of thigh. Middle: After application of fish skin. Right: Donor site fully re-epithelialized.
The quality of donor site healing was judged to be good in all cases (Fig. 2).
PARTIAL THICKNESS BURNS
We have used the fish skin as a treatment for partial thickness burns in selected patients, including a cooking-oil burn to the thigh (Fig. 3) and a flame injury on a hand (Fig. 4). Neither of these cases required skin grafting so were not included in the donor site study. Both of the wounds were completely epithelialized at the 2-week follow-up. Furthermore, the patients found the fish skin to be comfortable and have an immediate analgesic effect. Several patients noticed the “fish smell” of the product, but no-one strongly objected to this. The end results were esthetically pleasing, minimal scarring or color changes (Figs 3 and 4).
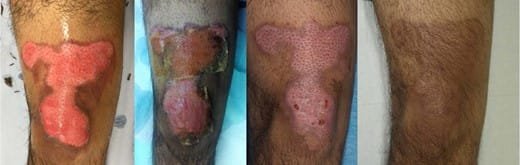
FIGURE 3.Healing sequence of a cooking oil deep partial thickness burn to lower part of anterior thigh and knee.
Left: Burn after debridement. Middle left: 1 week after application of fish skin. Middle right: Good progression of healing at 2 weeks. Right: Three months after injury wound is healed with excellent esthetic outcome.
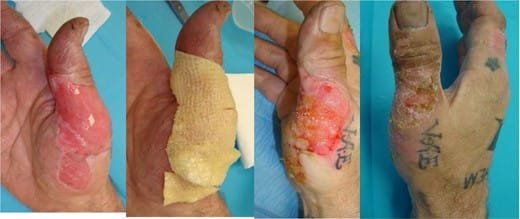
FIGURE 4.Healing sequence of a hand burn following flame injury.
Left: After burn debridement. Middle left: Application of fish skin. Middle right: Burn wound at 1 week, prior to reapplication of fish skin. Right: Follow-up at 2 weeks with a good healing outcome.
DISCUSSION
As this is a small case series we cannot make claims of effectiveness, which would require a more rigorous clinical trial. When compared to the literature it reflects favorably on the use of the fish skin graft. In the largest, multicenter randomized clinical trial that has been performed on various dressings to treat donor site wounds the median healing time was much higher. For the hydrocolloid dressing, which outperformed the other treatment modalities the average time to heal was 16 days.14 Any product that can shorten the healing times of donor site wounds can be immensely valuable under circumstances of a large surface area burn, necessitating frequent re-harvesting of donor site areas that are very limited in these cases.
The fish skin maintains its three-dimensional structure and is highly porous which provide an extracellular matrix composed of glycosaminoglycans, proteoglycans, fibronectin and growth factors15,16 which allows the migration of autologous cells to promote the proliferative and epithelialization phases of the burn healing process.15,17 The graft contracts slightly after salination and insertion in the wound bed, so it is recommended that pre-wetting takes place before the product is applied so that any shrinkage occurs before application to the patient. A small area of overlap should be made, as is normal when applying any dressing, to allow for any slight slippage.
CONCLUSIONS
This is the first study to show the effectiveness of using fish skin in acute burns. The experience in our center for partial thickness burns is promising. The healing outcomes for the treatment of donor site wounds are also highly encouraging, especially when compared to other RCTs on other products (Fig. 5 and Table I).14,18–22 One of the weaknesses of this study is that this was a pilot case series with a low number of patients. Both the analgesic effect noted and the relatively short average times until 100% re-epithelialization are promising and need to be studied further in a randomized controlled trial comparing the fish skin with current standard of care.
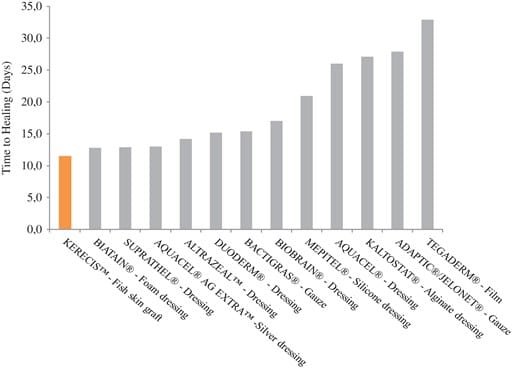
FIGURE 5.Comparison of results in days until healing when using fish skin on donor site wounds in this case series with published literature.
Presented as an oral presentation at the 2017 Military Health System Research Symposium.
References
- Puri V, Khare NA, Chandramouli MV, Shende N, Bharadwaj S: Comparative analysis of early excision and grafting vs delayed grafting in burn patients in a developing country. J Burn Care Res 2016; 37(5): 278–82.
- Moss A Jr, Fryer JP, Leventhal JR, et al. : Analysis of the human anti-pig cellular immune response using the Hu-scid mouse-porcine skin graft model. Transplant Proc 1994; 26(3): 1209.
- Yang CK, Polanco TO, Lantis JC 2nd: A prospective, postmarket, compassionate clinical evaluation of a novel acellular fish-skin graft which contains omega-3 fatty acids for the closure of hard-to-heal lower extremity chronic ulcers. Wounds 2016; 28(4): 112–8.
- Magnusson S, Baldursson BT, Kjartansson H, Rolfsson O, Sigurjonsson GF: Regenerative and antibacterial properties of acellular fish skin grafts and human amnion/chorion membrane: implications for tissue preservation in combat casualty care. J Mil Med 2017; 182(S1): 383–8.
- Baldursson BT, Kjartansson H, Konradsdottir F, Gudnason P, Sigurjonsson GF, Lund SH: Healing rate and autoimmune safety of full-thickness wounds treated with fish skin acellular dermal matrix versus porcine small-intestine submucosa: a noninferiority study. Int J Low Extrem Wounds 2015; 14(1): 37–43.
- Trinh TT, Dunschede F, Vahl CF, Dorweiler B: Marine Omega3 Wound Matrix for the treatment of complicated wounds. Phlebologie 2016; 45: 93–8.
- Rakers S, Gebert M, Uppalapati S, et al. : “Fish matters”: the relevance of fish skin biology to investigative dermatology. Exp Dermatol 2010; 19(4): 313–24.
- Magnusson S, Baldursson BT, Kjartansson H, et al. : Decellularized fish skin: characteristics that support tissue repair. Laeknabladid 2015; 101(12): 567–73.
- Serhan CN: Pro-resolving lipid mediators are leads for resolution physiology. Nature 2014; 510(7503): 92–101.
- Trinh TT, Dünschede DF, Vahl C-F, Dorweiler B:: Marine Omega3 Wound Matrix for the treatment of complicated wounds. Phlebologie 2016; 45: 93–8.
- Dorweiler B, Trinh TT, Dünschede F, et al. : Die marine Omega-3-Wundmatrix zur Behandlung komplizierter Wunden. Gefässchirurgie 2017; 22(8): 558–67.
- McDaniel JC, Belury M, Ahijevych K, Blakely W: Omega-3 fatty acids effect on wound healing. Wound Repair Regen 2008; 16(3): 337–45.
- Lantis JC, Sigurdardottir RS, Haflidadottir IL, et al. : Fish skin Omega3’s induce cell migration and transcription of ALOX15 – a specialized pro-resolving mediator forming lipoxygenase. Symp Adv Wound Care Fall Meet Oct 20–22 Las Vegas Poster Present. 2017.
- Brölmann FE, Eskes AM, Goslings JC, et al. : Randomized clinical trial of donor-site wound dressings after split-skin grafting. Br J Surg 2013; 100(5): 619–27.
- Armstrong RB, Nichols J, Pachance J: Punch biopsy wounds treated with Monsel’s solution or a collagen matrix. A comparison of healing. Arch Dermatol 1986; 122(5): 546–9.
- Richardson R, Metzger M, Knyphausen P, et al. : Re-epithelialization of cutaneous wounds in adult zebrafish combines mechanisms of wound closure in embryonic and adult mammals. Development 2016; 143(12): 2077–88.
- Badylak SF: The extracellular matrix as a scaffold for tissue reconstruction. Semin Cell Dev Biol 2002; 13(5): 377–83.
- Markl P, Prantl L, Schreml S, Babilas P, Landthaler M, Schwarze H: Management of split-thickness donor sites with synthetic wound dressings: results of a comparative clinical study. Ann Plast Surg 2010; 65(5): 490–6.
- Assadian O, Arnoldo B, Purdue G, et al. : A prospective, randomised study of a novel transforming methacrylate dressing compared with a silver-containing sodium carboxymethylcellulose dressing on partial-thickness skin graft donor sites in burn patients. Int Wound J 2013; 12(3): 351–6.
- Solanki NS, Mackie IP, Greenwood JE: A randomised prospective study of split skin graft donor site dressings: AWBAT-D vsDuoderm®. Burns 2012; 38(6): 889–98.
- Kaiser D, Hafner J, Mayer D, French LE, Lauchli S: Alginate dressing and polyurethane film versus paraffin gauze in the treatment of split-thickness skin graft donor sites: a randomized controlled pilot study. Adv Skin Wound Care 2013; 26(2): 67–73.
- Bailey S, Carmean M, Cinat M, Burton K, Lane C, Malinoski D: A randomized comparison study of Aquacel Ag and Glucan II as donor site dressings with regard to healing time, cosmesis, infection rate, and patient’s perceived pain: a pilot study. J Burn Care Res 2011; 32(6): 627–32.